
The ArmonAir Digihaler may help you
Review quality of inhalations and detect missed doses3*†
Indicated for the maintenance treatment of asthma as prophylactic therapy in patients 12 years of age and older.
Limitation of Use: ArmonAir Digihaler is not indicated for the relief of acute bronchospasm.
Contraindicated in the primary treatment of status asthmaticus or other acute episodes of asthma requiring intensive measures. Contraindicated in patients with known severe hypersensitivity to milk proteins or any ingredients of ArmonAir Digihaler.
Please see additional Important Safety Information in the scroll below.
*As measured by inspiratory flow.
†Inhaler use is recorded as an event when a patient opens the cap or inhales.
See the Data
ArmonAir Digihaler
- Dosing
- Features
- Efficacy
- Adverse Events
ArmonAir Digihaler has the lowest approved dose of fluticasone propionate at each strength3,11,12
| ArmonAir Digihaler | Flovent® HFA | Flovent® Diskus® | |||
| Available Strengths | 55 mcg 113 mcg 232 mcg |
44 mcg 110 mcg 220 mcg |
50 mcg 100 mcg 250 mcg |
||
| Low | 55 mcg | 88 mcg | 100 mcg | ||
| Medium | 113 mcg | 220 mcg | 250 mcg | ||
| High | 232 mcg | 440 mcg | 500 mcg |
Flovent® HFA and Flovent® Diskus® are registered trademarks of the GSK group of companies.
ArmonAir Digihaler should be given3:
- As 1 inhalation twice daily about 12 hours apart
- By oral inhalation only
Table is intended to show differences in dosing only and should not be construed to imply safety or efficacy similarities or differences.
Abbreviation: HFA=hydrofluoroalkane
ArmonAir Digihaler vs nondigital counterpart
| Features | ArmonAir® Digihaler® | ArmonAir RespiClick® |
| Efficacy and safety3 | Digihaler inhalers and their nondigital counterparts share the same efficacy and safety profile* | |
| Tracks and categorizes quality of inhalations3 | ||
| Measures inspiratory flow rates3 | ||
| Tracks how often the inhaler is used3-5 | ||
| Captures patterns of maintenance inhaler use and missed doses3-5 | ||
| Connects to a companion mobile app for viewing inhaler use data3 | ||
Inhaler use is recorded as an event when
a cap is opened or a patient inhales.
* The Digihaler products were approved based on the safety and efficacy data from their nondigital counterparts.
Product characteristics listed are not all-inclusive and cannot be used to infer product efficacy or safety
Connection to the app is required for transmission of data, but is not required for delivery of the medicine from the inhaler.
The safety of AirDuo Digihaler has been established from adequate and well-controlled studies of fluticasone propionate and salmeterol MDPI. There is no evidence that the use of the Digihaler app leads to improved clinical outcomes, including safety and effectiveness.
Improvements in lung function in a clinical trial program3
Fluticasone propionate MDPI demonstrated significantly greater FEV1 improvement vs placebo across all dosage strengths.
Trial 1: Mean change from baseline in trough FEV1 at each visit by treatment group (FAS)
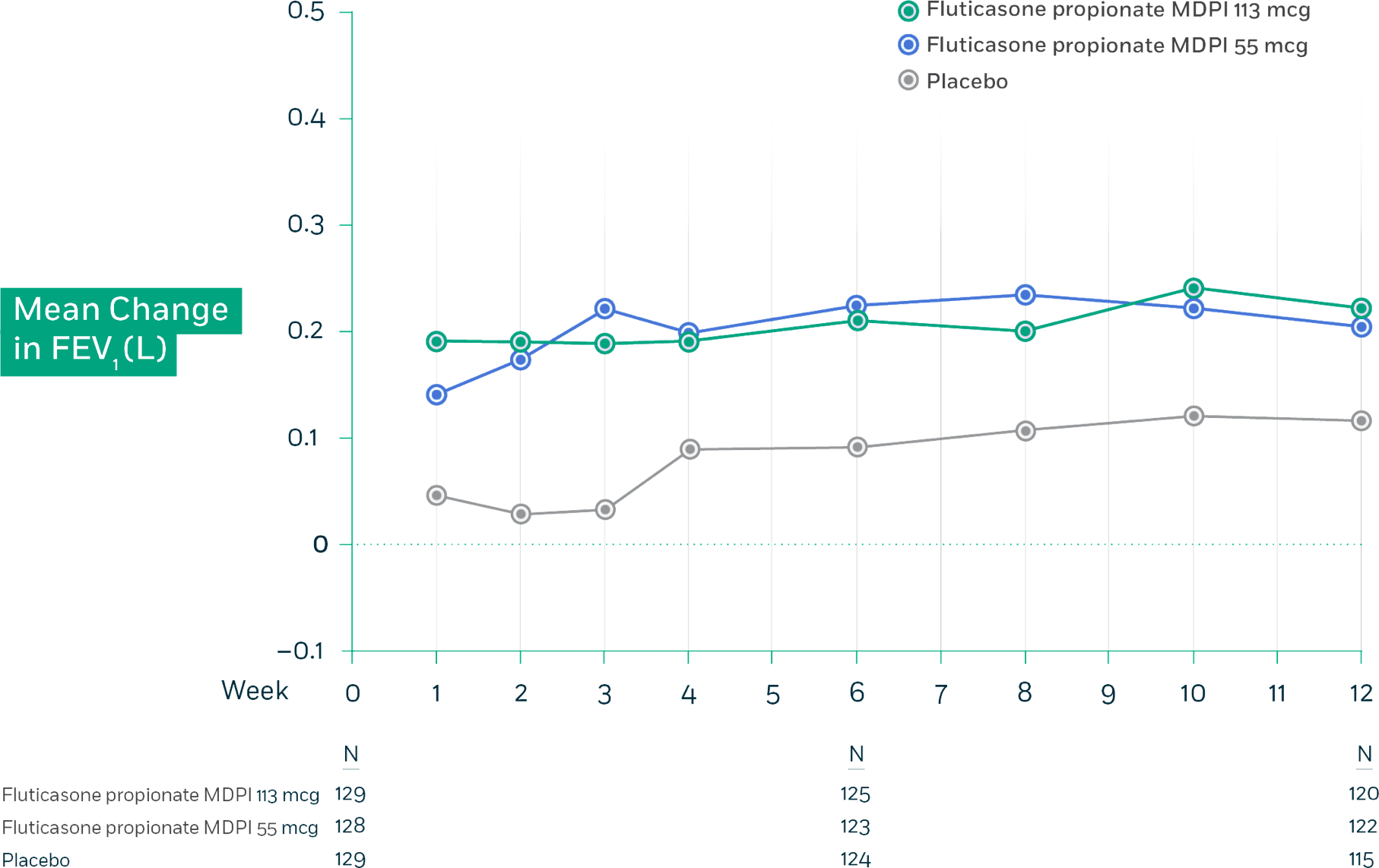
Trial 1 Design: This randomized, double-blind, placebo-controlled, 12-week, global efficacy and safety trial compared fluticasone propionate MDPI 55 mcg and 113 mcg (1 inhalation twice daily) and placebo in adolescent and adult patients with persistent symptomatic asthma despite low-dose or mid-dose ICS or ICS/LABA therapy. Additional arms in this study received fluticasone propionate and salmeterol MDPI 55/14 mcg and 113/14 mcg (1 inhalation twice daily). The primary endpoint for this trial was the change from baseline in trough FEV1 at week 12 for all patients.
Fluticasone propionate MDPI demonstrated significantly greater FEV1 improvement vs placebo across all dosage strengths.
Trial 2: Mean change from baseline in trough FEV1 at each visit by treatment group (FAS)
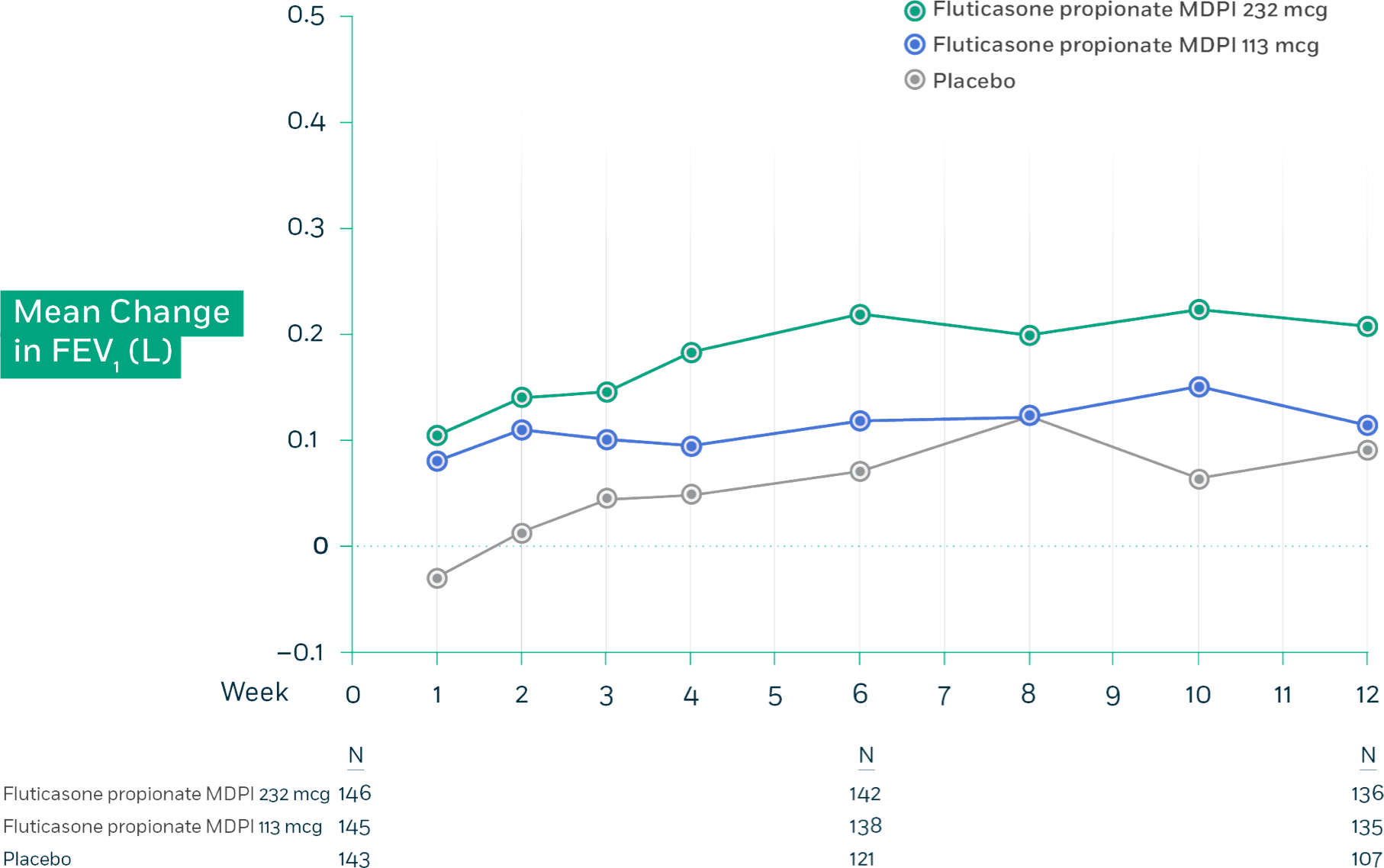
Trial 2 Design: This randomized, double-blind, placebo-controlled, 12-week, global efficacy and safety trial compared fluticasone propionate MDPI 113 mcg and 232 mcg (1 inhalation twice daily) and placebo in adolescent and adult patients with persistent symptomatic asthma despite ICS or ICS/LABA therapy. Additional arms in this study received fluticasone propionate and salmeterol MDPI 113/14 mcg and 232/14 mcg (1 inhalation twice daily). The primary endpoint for this trial was the change from baseline in trough FEV1 at week 12 for all patients.
Abbreviations: FAS=full analysis set; FEV1=forced expiratory volume in 1 second; ICS=inhaled corticosteroid; LABA=long-
acting beta2-agonist; MDPI=multidose dry powder inhaler
Safety profile3
The safety of fluticasone propionate MDPI was evaluated in patients with asthma who were
previously treated with an ICS.
Adverse reactions with ≥3% incidence with fluticasone propionate MDPI, and more common
than placebo in subjects with asthma in trials 1 and 2.
| Flucticasone propionate MDPI | ||||
| Adverse Reaction | 55 mcg (n=129) % |
113 mcg (n=274) % |
232 mcg (n=146) % |
Placebo (n=273) % |
| 5.4 | 5.8 | 4.8 | 4.4 | |
| URTI | 5.4 | 4.7 | 5.5 | 4.8 |
| Oral candidiasis* | 3.1 | 2.9 | 4.8 | 0.7 |
| Headache | 1.6 | 7.3 | 4.8 | 4.4 |
| Cough | 1.6 | 1.8 | 3.4 | 2.6 |
*Oral candidiasis includes oropharyngeal candidiasis, oral fungal infection, and oropharyngitis fungal.
In a long-term, open-labeled safety study, the types of adverse reactions were similar to those reported above in the placebo-controlled studies. The 26-week safety study included 674 patients previously treated with ICS who were treated twice daily with fluticasone propionate MDPI 113 mcg or 232 mcg; fluticasone propionate/salmeterol MDPI 113/14 mcg or 232/14 mcg; fluticasone propionate inhalation aerosol 110 mcg or 220 mcg; fluticasone propionate and salmeterol inhalation powder (250/50 mcg), or fluticasone propionate and salmeterol inhalation powder (500/50 mcg), the types of adverse reactions were similar to those reported above in a placebo-controlled study.
Abbreviation: URTI=upper respiratory tract infection

