
EXPLORE A COLLECTION OF
Resources and support
- Request A Rep
- For Your Practice
- For Your Patients
Request a rep
Complete the fields below to be contacted by a Digihaler representative.
All fields required.
Thank you for submitting your request.
A digihaler representative will be in contact with you.
There was an error trying to send your message.
Please try again later.
LEARN MORE
Hear from your peers
See what other healthcare professionals have to say about the Digihaler family of inhalers.




Explore the Digihaler Dashboard
A secure, online tool that enables you to access multiple patients' inhaler-event data to maximize your experience with the Digihaler system.*†
Learn More*Inhaler use is recorded as an event when the cap is opened or a patient inhales.
†Data can be viewed if the patient chooses to share it with you.
Digihaler Savings Program
Help patients pay less*
Most commercially insured patients may be eligible for the savings program.†
Learn more*For AIRDUO® DIGIHALER® (fluticasone propionate and salmeterol) and ARMONAIR® DIGIHALER® (fluticasone propionate) only.
†Please note, this offer is not valid for patients eligible for Medicare, Medicaid, or any other form of government insurance. See full terms and conditions.
Helping patients connect their Digihaler device to the app
Your patients must first download the Digihaler app and pair their inhaler in order for you to
review their inhalation data.
Walk them through these 3 basic steps:
Download
- Patients download the app through the App
Store or Google Play on their smartphone -

Register
- Patients open the app and
follow the on-screen instructions to
set up their account
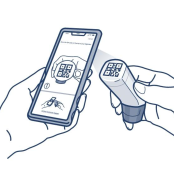
Scan
- Patients find the black QR code on the top of their inhaler
- In the app, they tap the “scan” button
- Patients center the QR code on their inhaler in the camera’s viewfinder
- When the inhaler is registered with the app, their screen will say “Your Digihaler is registered”
Patients should remember to keep their Bluetooth on so that Digihaler will always be able to capture their data. If they
have any issues, live agents are waiting to help at 877-410-DIGI (3444).
Share the Getting Started video with your patients

DOWNLOAD ADDITIONAL INFORMATION
Other resources
Digihaler Welcome Brochure
The Digihaler Portfolio Welcome
Brochure can help patients learn
more about the Digihaler family of
inhalers and the Digihaler app.
Patient Quick Start Guide
A guide to help your patients
download the app, register
for
a Digihaler account, and pair
their inhaler.
Pre-Authorization Resources
For prescribers
If prior authorization is required, the following resources, documents,
and templates may help your patients
Letter of Medical Necessity Template
Use this template to write a letter to establish medical necessity, which payers may require for treatment.
ProAir Digihaler AirDuo Digihaler ArmonAir DigihalerFormulary Letter of Appeals Template
Use this template to write an appeals letter, which
payers may need to
appeal a denial of coverage for treatment.
ArmonAir Digihaler
Pre-Authorization Resources
For pharmacists
If prior authorization is required, the following resources, documents,
and templates may help your patients
Pharmacist Digihaler® savings program processing instructions
Use these instructions when
processing the Digihaler savings program.
